Vanadium chrysoberyl
First published in Facette 29 (May 2024)
Vanadium as a colouring element is quite often underestimated, mostly as it is often associated and acting together with chromium, a chemical element with a similar geochemical behavior and paragenetic occurrence but which is historically better known in the trade and easier to detect with simple tools (spectroscope) by gemmologists.

Apart from tanzanite (V-bearing zoisite) and tsavorite (V-bearing grossular garnet), vanadium is actually also found as an important chemical constituent for example in chrome-tourmaline – which is in fact mostly dominated by vanadium despite its trade name – and Colombian emeralds. In all these cases, vanadium is the main cause of or at least contributing to the colour of these gems.
Vanadium-chrysoberyl (or V-chrysoberyl) is an interesting and very attractive addition to these vanadium-coloured gems. Its colour ranges from bright ‘mint’ green to saturated bluish green (Figure 1). It has been found in Tunduru (Tanzania), Ilakaka (Madagascar), Sri Lanka and Myanmar (Krzemnicki & Kiefert, G&G 1996, Schmetzer et al. JoG 2013, see also SSEF Facette 2014, page 20). Being rather rare but often nearly free of inclusions, these gems are sought after by gemstone collectors and jewellery designers.
At SSEF, the classification as vanadium chrysoberyl is based on a combination of visual appearance (colour), absorption spectroscopy and chemical trace element analysis, very similar to other colour variety calls, such as cobalt-spinel, emerald, Paraiba tourmaline, to name a few.
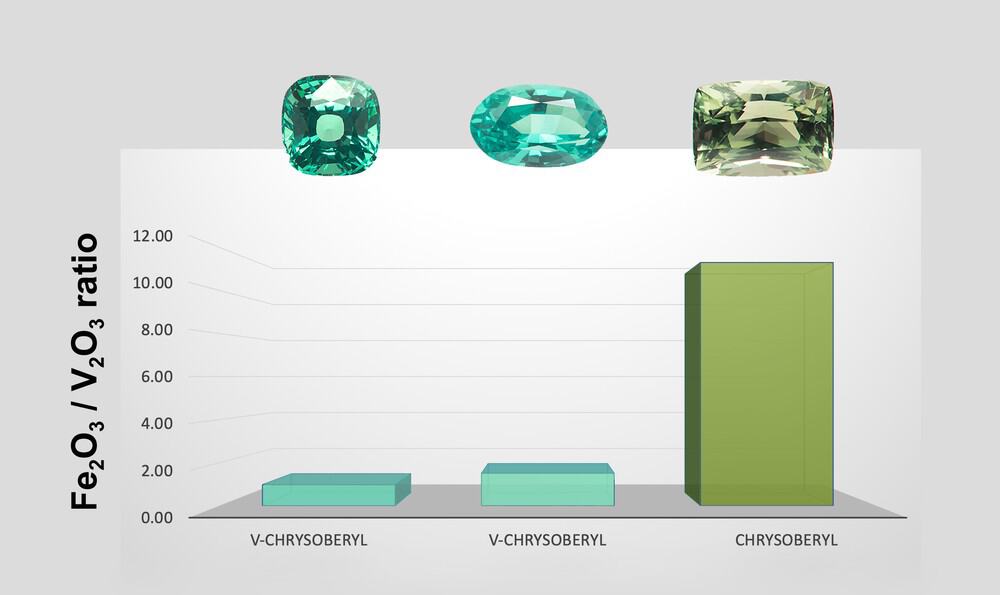
It is important to understand that yellowish green chrysoberyl mainly coloured by iron with traces of vanadium is not fitting to be classified as V-chrysoberyl. As there is a gradual range between vanadium chrysoberyl and iron-dominated chrysoberyl, a separation into these two varieties is not always straightforward and requires a standardized analytical protocol and light (see also the light box article, page 44). The complexity is further increased as there are two different lattice sites in the chrysoberyl structure on which vanadium, but also Cr and Fe may substitute for aluminium, resulting in slightly different impact on absorption and finally colour. Ultimately, the visual colour, i.e. the predominance of a ‘mint’ green to bluish green colour is key to recognize and appreciate this attractive colour variety of chrysoberyl.